60
- Labtests Online UK
- Better Testing.org
- Leeds Teaching Hospitals Pathology Services Clinical Support
- Leeds Test & Tubes Database
- MD Calc
- MIMS Clinical Calculators
- Clinical Biochemistry Rotherham
- Uncommon tests and Procedures Rural Health West au
| Normal values | |
|---|---|
| Acid Phosphatase | |
| Albumin | 35-50 g/l |
| Alkaline Phosphatase | 30-130 U/l |
| AFP | |
| Ammonia | |
| Amylase | |
| ASO | |
| B12 | |
| Base Excess | |
| Bicarb | |
| Bilirubin | |
| Bleeding Time | |
| BUN | |
| C peptide | |
| CRP | |
| Ca | |
| CO2 | |
| CEA | |
| Chloride | 95-108 mmol/l |
| Cholesterol | |
| Cold Agglutins | |
| C3 Complement | |
| C4 Complement | |
| C50 Complement | |
| Coombs direct | |
| Coombs indirect | |
| Cortisol | |
| CPK | |
| Creatinine | |
| Faecal Fat | |
| FDPs | |
| Fibrinogen | |
| Folic Acid | |
| FTA abs | |
| Glucose | |
| HbA1c | |
| HbsAg | |
| Haptoglobulin | |
| beta HCG | |
| Iron | |
| TIBC | |
| LDH | |
| Lactic Acid | |
| LAP | |
| LEE White Clotting Time | |
| LE | |
| Lipase | |
| Mg | 0.7-1.0 mmol/l |
| Monospot | |
| 5 nucleotidase | |
| Osmolality | |
| paO2 | |
| PTT | |
| P04 | 0,8-1.5 mmol/l |
| K | 3.5-5.3 mmol/l |
| Protein | |
| PT | |
| RhF | |
| ESR | |
| GGT | |
| SGOT | |
| SGPT | |
| Na | 133-146 mmol/l |
| FOB | |
| Sweat Test | |
| TSH | |
| T3 | |
| Total T4 | |
| Free T4 | |
| TBG | |
| Thrombin Time | |
| Transferrin | |
| TG | |
| TORCH | |
| Troponin | |
| Uric Acid | 200-400 micomol/l male140-360 micromol/l female |
| VDRL | |
| Hb | |
| WCC | |
| Platelets | |
| Haematocrit | |
| Reticulocytes | |
| Left Shift | |
| MCV | |
| MCHC | |
| Haematocrit | |
| Basophils | |
| Esosinophils | |
| Lymphocytes | |
| Monocytes | |
| Crisis lab values sodium | ||
|---|---|---|
| low | < 120 mmol | diureticsCCF |
| high | > 160 mmo | dehydrationcollapse |
| Crisis lab values potassium | ||
|---|---|---|
| low | < 3 mmol/l | D+V diureticscardiotoxicity arrest |
| high | > 6 mmol/l | renal disease diureticscardiotoxicity arrythmia |
| Crisis lab values calcium | ||
|---|---|---|
| low | < 1.5 mmol/l | vit D/PTH deficiency tetany fits |
| high | > 3.2 mmol/l | hyperparathyroidism coma |
| Crisis lab values bicarbonate | ||
|---|---|---|
| low | < 10 mmol/l | renal failure |
| high | > 40 mmol/l | hepatic failureReyes GIB CCF |
| Crisis lab values ammonia | ||
|---|---|---|
| low | <8.8 micromol/l | renal failure |
| high | >29.3 micromol/l | hepatic failureReyes GIB CCF |
| Crisis lab values glucose | ||
|---|---|---|
| low | < 2.2 mmol/l | |
| high | > 16.6 mmol/l | |
| Crisis lab values PaO2 | ||
|---|---|---|
| low | < 6.7 kPa | |
| high | ||
| Crisis lab values PaCO2 | ||
|---|---|---|
| low | < 2.7 kPa | |
| high | > 9.3 kPa | |
| Crisis lab values pH | ||
|---|---|---|
| low | < 7.2 | |
| high | >7.6 | |
| Crisis lab values Hb | ||
|---|---|---|
| low | < 80 g/l | |
| high | > 18 g/l | |
| Crisis lab values platelets | ||
|---|---|---|
| low | < 50 k/mm3 | bone marrow suppressionhaemorrhageITP |
| high | > 500 k/mm3 | leukaemiareaction to acute bleed |
| Crisis lab values INR | ||
|---|---|---|
| >3overanticoagulation DIC | ||
| high | ||
| Crisis lab values PT | ||
|---|---|---|
| x | ||
| high | >14 sec (>20 sec on warfarin)anticoagulant therapy factor deficiencybleeding | x |
| Crisis lab values PTT | ||
|---|---|---|
| high | >40 sec (>70 sec on heparin) | factor deficiencybleeding |
Haematology
| Full Blood Count FBC | |
|---|---|
| Hb | 130-180 g/l male 120-160 g/l female |
| Hct | 45-54 ml/dl male 37-47 ml/dl female |
| MCV | 80-100 fl |
| WCC | 4.5-11 x 10-9/L |
| Platelets | 150-400 x 10-9/L |
| Lymphocytes | 25-33% |
| Monocytes | 3-7% |
| Eosinophils | 3% |
| Peripheral Blood Film RBC/WBC morphology | |
|---|---|
| poikilocytosis | |
| anisocytosis | |
| basophilic stippling | |
| Howell-Jolly Bodies | |
| Sickling | |
| Nucleated RBCs | |
| Target Cells Leptocytes | |
| Spherocytes | |
| Helmet Cells Schistocytes | |
| Burr Cells Acanthocytes | |
| Polychromasia | |
| Auer Rod | |
| Dohle Bodies | |
| Hypersegmentation | |
| Toxic Granulation | |
low Hb / anaemia
- Anaemia Basic Tests YT
- Complex Review Anaemia YT
- RBC lifecycle YT
- Increased RBC loss/destruction YT
- Decreased RBC Production YT
- Pulse Learning Anaemia
Microcytic / Fe deficiency anaemia
- Microcytic anaemias Comlex YT
- Fe defficiency Anaemia YT
- Fe deficiency anaemia @ BetterTesting.org.uk
- Investigating iron status in microcytic anaemia Sep 2006 BMJ
- GP Online
Patients over 40 with iron deficiency anaemia should be investigated from a gastroenterological viewpoint, to exclude occult malignancy.
Iron supplementation (bd or tds, ideally with vitamin C to increase absorption) should lead to a 1 g/dl rise in Hb per week and should be continued for at least 3 months after the Hb comes back to normal.
Ferritin levels of >25 glml in the absence of anaemia are unlikely to explain symptoms of tiredness and do not require treatment.
It is worth noting that ferritin itself is an inflammatory marker; iron studies may therefore be easier to interpret than ferritin level
| Macrocytic anaemia / macrocytosis |
|---|
| B12 / folate deficiency B12 is sometimes given orally at doses of 1-2 mglday if there is a dietary deficiency (unlicensed use), but, if not, should be administered by intramuscular injection (three times a week for the first 2 weeks, then once every 3 months). B12 levels are invariably high in patients receiving injections. Low folate levels can result from a poor diet (eg in alcoholics), increased need (eg in pregnancy, haemodialysis and haemolytic anaemias) and malabsorption (eg coeliac disease). latrogenesis may also be a cause, eg in patients taking folate antagonists such as phenytoin or methotrexate. Folic acid should not be given if there is concurrent vitamin B12 deficiency until the patient has had at least one injection of vitamin B12; otherwise, subacute combined degeneration of the spinal cord can be precipitated or worsened. When treating folate deficiency, folic acid should be given at a dose of 5 mg per day for 4 months |
| Drugs, including alcohol, azathioprine and AZT |
| Hypothyroidism |
| Liver disease |
| Myelodysplasia |
| Marrow infiltration |
| Haemolysis |
| Pregnancy |
Investigations
liver function tests
urea and electrolytes
thyroid function tests
B12
folate
reticulocyte count
When an underlying cause is not obvious, persistant macrocytosis should be referred to the haematologists, to exclude a malignancy such as myelodysplasia
Normocytic anaemia
Normocytic anaemia – first check the haematinics
if the patient is iron deficient, they may be losing blood slowly and should be investigated from a gastroenterological point of view.
If the haematinics are normal chronic disease (eg renal failure) and haematological malignancy should be excluded, eg myelodysplasia in the elderly.
Haemanitics / Iron Studies
Polycythaemia
Polycythaemia – High Hb / PCV / Haematocrit
Check whether Hb is getting progressively higher, whether the patient is symptomatic (eg headaches, dizziness, tingling, splenomegaly) and whether any of the other full blood count (FBC) parameters are abnormal (eg platelets or white cell count), possibly indicating the onset of myelofibrosis or leukaemia. (Urgent referral)
Dehydration can cause a raised Hb.
If both Hb and PCV are raised, repeat the FBC in a few weeks when the patient is well hydrated if still raised refer particularly for persistently raised Hb of >16 g/dl or PCV >0.48 in a woman and Hb >17.5 gidl or PCV >0.51 in a man
Only blood volume studies can absolutely determine whether there is a true increase in red cell mass; these are only done in a few specialist centres.
| Haemolytic anaemias | |
|---|---|
| Inherited | Red cell membrane defects – spherocytosis and elliptocytosisHaemoglobinopathy – SCD thalassaemiaMetabolic Defects -G6PD and pyruvate kinase deficiency |
| Infections | malaria, brucellosis endocarditis mycoplasma clostridium welchii |
| Drugs | penicillin cefalosporins sulphonamides rifampicin |
| Mechanical | Prosthetic valvesmalignant hypertensionDIC |
| Autoimmune | idiopathicunderlying diseasedrug relatedcold agglutinins |
| Hypersplenism | |
| Burns | |
| Transfusion reaction | |
- Haemolytic Anaemias Kaplan YT
- Hemolytic Anaemias YT
- Haemolytc Anaemia YT
- Chronic Haemolytic Anemia in SCD YT
- Haemolytic Anaemias medic8.com
- Haemolytic Anaemias Clare Murphy BMS2 Essex.ac.uk
Reticulocyte count
Reticulocytes Clinical Methods NCBI Bookshelf
Red cell morphology
| White cell count Never Let Momma Eat Beans (60, 30, 6, 3, 1) | |
|---|---|
| Neutrophils | 60% |
| Lymphocytes | 30% |
| Monocytes | 6% |
| Eosinophils | 3% |
| Basophils | 1% |
| White Blood Cell functions | |
|---|---|
| neutrophil | x |
| eosinophil | x |
| basophil/mast cell | x |
| monocyte | x |
| macrophage | x |
| lymphocyte | x |
| dendritic cell | x |
| natural killer cell | x |
Polymorphs / Neutrophils / Granulocytes
| Neutropaenia |
|---|
| viral infections |
| fulminant bacterial infection (including military TB) |
| low normal – sometimes, they have had a low neutrophil count for years, but are perfectly well in themselves, in which case it is very unlikely that there is a sinister underlying cause |
| racial variation Certain racial groups, eg black Africans, are more likely to have low neutrophil counts; a count of 1.8×1 09 /1 is probably normal in these groups |
| drug-induced |
| autoimmune neutropenia (this is really a diagnosis of exclusion based on the finding of a normal bone marrow) |
| SLE & Feltys |
| myelodysplasia – generalised pancytopenia, immediate referral |
Low neutrophils (normal range 2.0 x 109-7.5 X 109 cells/I)
If there is an isolated neutropenia, it is worth repeating a FBC in 4-6 weeks. If the neutropenia is progressive, again the patient should be referred. It is especially alarming if the patient reports that they have recently become more susceptible to picking up infections (this commonly occurs when the neutrophil count falls below 0.5 x 109 /1).
Eosinophils
- Eosinophils YT
- Eosinophil Granulocyte YT
- Eosinophil Mast Cell YT
- How should I interpret a raised eosinophil count? @ bettertesting.org.uk
Leukopaenia and agranulocytosis
- Neutropaenia Medscape
- Leukopaenia University Virginia
- Leukopaenia RightDiagnosis.com
- When should I refer a patient with a low neutrophil count? Bettertesting
Lymphocytes
- Lymphocytes YT
- Lymphocyte trafficking YT
- Lymphocyte YT
- When should I refer an adult patient with a lymphocytosis? bettertesting.org.uk
Monocytes
| Thrombocytopaenia |
|---|
| failure of platelet production |
| Failure of platelet production can occur due to drugs, alcohol abuse, viral infection, myelodysplasia and other haematological malignancies that result in bone marrow infiltration. Myelodysplasia should be considered if an elderly person has an isolated thrombocytopenia. |
| shortened platelet survival Shortened platelet survival occurs in idiopathic autoimmune thrombocytopenia (lTP). ITP usually presents with petechiae, bruising and spontaneous bleeding from mucous membranes. |
| Acute ITP most commonly children under 10 years. Almost always self-limiting, usually within 2-4 weeks. Platelet counts are often <20 xl 09 /1. Steroids and iv immunoglobulin may be used but > than 80% of patients will recover without treatment. |
| Chronic ITP most common in the 15 to 50-year-old age group. Platelet count is usually between 20 x 109 and 80 x 109 /1 Relapsing and remitting, with spontaneous cure being rare. If the platelet count is >30 x 109 /1 and the patient is asymptomatic, then treatment is not usually necessary. If treatment is required, it is usually with oral prednisolone; if there is poor steroid response, splenectomy is sometimes necessary. Patients with chronic ITP should be referred urgently if they are actively bleeding (at any platelet count) or if the platelet count is below 5 x 109 /1. Thrombopoietin-Receptor Agonists for Primary Immune Thrombocytopenia NEJM 2011 |
Thrombocytosis
can occur as a result of any physiological stress; for example, trauma, surgery, infection, inflammatory disease (eg rheumatoid arthritis and ulcerative colitis), malignancy and iron deficiency anaemia.
It can also be due to myeloproliferative disease such as essential thrombocythaemia, which is more common in the elderly and can present with thrombotic or bleeding complications.
In general, early referral should be made if other FBC parameters are also raised, or if the platelet count is >600 x 109 /1.
PV CRP ESR
ESR (men) = age/2
ESr (women)= age +10/2
- Inflammatory markers BMJ Feb 2012
- ESR bettertesting.org.uk
- Leeds Teaching Hospitals Apr 2009 PV, ESR or CRP?
- CRP and procalcitonin in Febrile Children InfoPOEMS Aug 2011
- ESR YT
- ESR YT
- Understanding ESR
- ESR YT
- ESR YT
Monospot
- youtu.be/hT8OHWcn4T4
- hacking-medschool/glandular-fever
- Tests for infectious mononucleosis bettertesting.org.uk
Clotting studies
| Coagulation Studies | ||
|---|---|---|
| ACT | 107 sec +/- 13 sec | |
| Bleeding Time | 3-6 mins | |
| D-dimer | <1.37 nmol/l | |
| Fibrinogen | 2-4 g/l | |
| Plasminogen | 80-130% | |
| PT | 10-14 secs | |
| PTT | 21-35secs | intrinsic pathway for patients on heparin (aka Kaolin Cephalin Clotting Time KCCT) |
| APPT | Do not confuse this with the Kaolin Clotting Time (KCT) which is a screening test for a lupus anticoagulant. Partial Thromboplastin Time with Kaolin (PTTK) |
|
| Thrombin Time | 10-15 secs | extrinsic pathway time taken for clotting of citrated plasma after addition of clacium and stanardised reference thromboplastin and expressed as the ratio of the patient’s PT to that of a pool of plasma taken from health subjects on no medication Prolonged when functional fibrinogen levels are <1g/l |
| INR | 2.0-3.0 | essentialy an internationally standardised form of the PT for patients on warfarin |
The division of the clotting cascade into intrinsic, extrinsic and common pathways has little in vivo validity but remains a useful concept for interpreting the results of laboratory investigations
| APTT |
|---|
| Isolated Prolonged APTT Deficiencies of either XII, XI, X, IX, VIII, V, II and fibrinogen. Contact factor deficiency e.g. pre-kallikrein deficiency [In multiple clotting factor deficiencies the APTT becomes prolonged with less severe reductions in factor levels] Acquired clotting factor inhibitors these are most commonly directed against FVIII and may occur as either autoantibodies or alloantibodies (in patients with severe Haemophilia A). Inhibitors against other clotting factors are rare but doccur e.g. Factor V |
| Prolonged APTT + Prolonged PT Vitamin K deficiency Liver disease due to: Malabsorption of vitamin K [a fat soluble vitamin] and therefore, decreased gamma carboxylation of the vitamin K dependent clotting factors Decreased synthesis of clotting factors An acquired dysfibrinogenemia due to changes in the sialic acid content of the fibrinogen. This is similar tthe effect seen in the newborn infant the so-called ‘fetal fibrinogen.’ Direct thrombin inhibitors including Hirudin and Argatroban DIC due tthe consumption of clotting factors Massive blood transfusion leading ta dilutional coagulopathy In patients receiving thrombolytic therapy, the APTT may be prolonged due to reduction in fibrinogen In multiple clotting factor deficiencies the APTT becomes prolonged with less severe reductions in factor levels |
| Increased APTT± Prolonged PT Unfractionated heparin Antiphospholipid antibodies Acquired clotting factor inhibitors e.g. FV, FX |
| Prolonged PT ± Prolonged APTT Warfarin [the APTT may be only prolonged by a few seconds in patients whare stably anticoagulated on warfarin but in patients whare overdosed the APTT may be significantly prolonged |
| Short APTT 1. An acute phase response leading thigh FVIII levels see also Comment 6 below. 2. Difficulties in the collection of samples leading tactivation of coagulation within the collection tube. 3. A deficiency of factor XIII does not prolong the APTT or the PT. |
| The APTT may be used for the detection of antiphospholipid antibodies [i.e. lupus anticoagulant] but in these cases reagents that are known tbe sensitive ta lupus anticoagulant and frequently employing low concentrations of phospholipid, must be used. |
| The APTT is frequently used tmonitor patients receiving unfractionated heparin [UFH.] However, the APTT is very sensitive the levels of FVIII which is an acute phase protein. If the FVIII levels are raised then the APTT may be misleadingly short and not accurately reflect the degree of anticoagulation. In these cases, anti-Xa assays should be performed tmonitor anticoagulation. |
| The APTT forms the basis for a number of factor assays including Factors VIII, IX, XI and XI. Factors II, V and X can alsbe assayed using an APTT-based system although they are more commonly assayed using a 1-stage PT-based assay. |
| The APTT is used tscreen for the presence of a number of clotting factor inhibitors including FVIII and FIX. |
| In patients receiving very high concentrations of unfractionated heparin e.g. during cardiopulmonary bypass, the APTT will be unclottable. In these cases, monitoring of the degree of heparinisation is undertaken using a different assay usually the Activated Clotting Time (ACT). |
| Fibrinogen assays 1.5-4.0g/l |
|---|
| Thrombin time 13-15s Prolonged when functional fibrinogen levels are <1 g/l Congenital deficiencies of fibrinogen |
| DIC Following thrombolytic therapy Liver disease Malignancy |
| Some anticoagulants will prolong the thrombin time |
| Elevated levels of Fibrin(ogen) Degradation Products (FDPs) These interfere with fibrin polymerisation and can at high concentration lead to prolonged thrombin time |
| Paraproteins May interfere with fibrin polymerisation leading ta prolonged thrombin time |
| Hypoalbuminemia This can result in a prolongation of both the thrombin time and the reptilase time. The prolongation appears tbe an in vitrphenomenon and can be corrected by raising the albumin concentration in vitrwhich corrects the prolonged thrombin and reptilase times. These patients dnot appear tbe at increased risk of bleeding and there is some evidence that they may have hyperaggregable platelets rendering them at increased risk of thrombosis. |
| Amyloidosis Prolongation of the Thrombin time and the Reptilase time has been observed in patients with amyloidosis due tthe inhibition of the conversion of fibrinogen tfibrin. |
| Following the use of bovine thrombin Patients exposed tbovine thrombin may develop inhibitors that prolong the bovine-based thrombin time. If the antibody cross-reacts with human thrombin, human-based thrombin times can alsbe prolonged. The Reptilase time is normal with these inhibitors. |
| Pathological anticoagulants Heparin-like anticoagulants have been reported (rarely) in patients with malignancies or other disorders, leading ta prolonged thrombin time but a normal Reptilase time. |
| Hyperfibrinogenaemia Hyperfibrinogenaemia can on occasion be associated with a prolonged thrombin time (and reptilase time). The mechanism is unclear but may reflect interference with fibrin assembly by excess fibrinogen |
| Fetal fibrinogen The thrombin time in the neonate is often prolonged due tthe presence of a fetal fibrinogen. |
| Fibrinogen defects may be quantitative (hypoor hyper-fibrinogenaemia) or qualitative (dysfibrinogenaemia). Inherited dysfibrinogenaemia is rare with only 250-300 patients reported worldwide but an acquired defect of fibrinogen function is more common, especially in liver disease when the fibrinogen molecule is excessively glycosylated impairing its activity. Elevated levels of fibrin degradation products (FDPs) alsimpair the action of fibrinogen.Fibrinogen levels are a useful as part of the investigation of a bleeding tendency or an unexplained prolongation of the APTT or PT. Elevated levels may correlate with increased risk of thrombosis in epidemiological studies although the significance in individual patients is unclear. |
| Fibrinogen levels are reduced in: DIC due the the consumption of clotting factors Liver disease due tdecreased synthesis. An abnormal fibrinogen may be alsbe found in patients with liver disease due tan abnormal (increased) sialic acid content Massive transfusion leading ta dilutional coagulopathy Hypofibrinogenaemia, afibrinogenaemia and dysfibrinogenaemia Following thrombolytic therapy In some patients following treatment with asparaginase |
| Fibrinogen levels are increased in: Increasing age Female sex, pregnancy, oral contraception In post-menopausal women Acute phase reaction Disseminated malignancyFibrinogen is an inflammatory marker, a bit like the erythrocyte sedimentation rate (ESR), and can be raised for similar reasons. It can be difficult to interpret. |
The fibrinogen level is usually interpreted in the light of other clotting tests such as the prothrombin time (PT) and activated thromboplastin time (APTT). If the clotting based fibrinogen assay is significantly reduced both APTT and PT will be prolonged, however, although this indicates hypofibrinogenaemia it does not exclude additional defects in the coagulation cascade such as may be found in disseminated intravascular coagulation [DIC].
Conversely, if the APTT and PT are prolonged but the clotting based fibrinogen assay is normal it suggests a defect higher up the clotting cascade and individual factor assays or a 50:50 mix may be helpful.
If the clotting based fibrinogen assay suggests reduced fibrinogen but there is nobvious reason for this and there is an appropriate clinical context (e.g. family history of bleeding diathesis, poor wound healing, umbilical stump bleeding) it may be useful tperform an immunological fibrinogen assay.
Bleeding Time
Prothrombin Time
KCCT
INR
Coagulation Cascade Haemostasis
- youtu.be/xNZEERMSeyM
- youtu.be/co6ar6vVp70
- youtu.be/MPGe-guZMqM
- youtu.be/8W0UzjmSPWc
- youtu.be/8b62hxLOsKY
- youtu.be/Mtz_6Bc2tas
| D Dimers |
|---|
| pulmonary embolism |
| deep vein thrombosis |
| disseminated intravascular coagulation |
| postop |
Specific degradation products released when the fibrinolytic system attacks the fibrin matrix of fresh venous thromboemboli.
Normal D-dimer levels implies that there is no fresh thromboembolic material undergoing dissolution in the deep veins or pulmonary arteries.
Useful for ruling out PE in patients with a low pre-test probability of PE or a non diagnostic V/Q scan
.A D-dimer below a certain cut point rules out PE with a high predictive value in patients with a low or moderate clinical probability
ABO blood types
hacking-medschool/abo-rhesus-compatibility
| Type | % population | surface antigens | plasma antibodies | Can receive blood from |
|---|---|---|---|---|
| AB | 7 | A and B | neither | AB A B O universal receiver |
| A | 40 | A | b | A O |
| B | 10 | B | a | B O |
| O | 43 | neither | both a and b | O universal donor |
| Agglutinogens | surface antigens on RBCs |
| Agglutinins | antibodies in the plasma |
| Agglutination | clumping together and lysis of donated cells following incompatible transfusion |
Rhesus +ve/ -ve
Rhesus -ve individuals will not have antibodies against the Rhesus antigen (present on RBCs of Rh +ve individuals) unless previously exposed.
If re-exposed the rhesus antibody will then attack the rhesus antigen.
Rhesus in pregnancy (values and tests)
Fluid balance
| Fluid Compartments 70kg male | ||
|---|---|---|
| Total Body Water | 42 l | 60% body weight |
| Intracellular | 28 l | 40% |
| Extracellular | 14 l | 20% |
| Plasma | 3.5 l | 5% |
| Interstitial | 10.5 | 15% |
| Total blood volume | 5.6 l | 8% |
| 1233045 If PIT | |
|---|---|
| 12 l | IF |
| 3 l | plasma |
| 30 l | intracelular |
| 45 l | total body water (60% TBW) |
Urea and electrolytes
| Electrolytes | mmol/l |
| Na | 134-146 |
| K | 3.4-5.0 |
| CL | 98-108 |
| Bicarb | 22-29 |
| Anion Gap | 8-16 |
| Osmolality (calculated) | 275-295 |
| Urea | 4.0-8.0 |
| Creatinine | 0.05-0.12 mmol/l ???? 50-100 micromol/l ??? |
| eGFR (Calculated) | >90 ml/min/1.73m2 |
| Ca | 2.15-2.6 |
| Ca Corrected | 2.15-2.6 |
| Phosphate | 0.80 -1.40 |
| Magnesium | 0.7-1.1 |
| glucose | 3.9-6.2 fasting |
| Electrolyte Disturbance | |
|---|---|
| High Na | confusion stupor coma muscle tremors seixures pulmonary and peripheral oedema |
| Low Na | |
| High K | |
| Low K | |
| High Ca | |
| Low Ca | |
| High Mg | |
| Low Mg | |
| High phosphate | |
| Low phosphate | |
Sodium balance
| Hyponatraemia |
|---|
| Drug-induced, in particular thiazide diuretics (especially combinations such as co-amilozide) |
| Overhydration Excess water intake (or fluid retention in cardiac failure or liver cirrhosis) |
| Fluid loss replaced with inadequte sodium |
| Addisons |
| Pneumonia in the elderly |
| Burns |
| Pseudohyponatraemia (if lipid levels are elevated or if there is a paraprotein) |
| Sodium loosing Nephritis |
| General Practice The Clinical Survival Guide/Endrocrinology |
Investigations
Measure plasma and urine osmolality (samples should be taken at approximately the same time) and urinary sodium.
Suspect SIADH if urine osmolality is>100 mosmollkg, plasma osmolality is <270 mosmol/kg and urinary sodium is >20 mmol/I, especially if the patient is not dehydrated or on diuretics.
If the urinary sodium is >20 mmol/I but the plasma and urine osmolalities are normal, there may be renal sodium loss, eg in analgesic nephropathy and polycystic kidney disease.
If the urine sodium is <20 mmol/l and the serum osmolality is reduced, consider excess water intake.
Management
In general, most patients are able to tolerate a sodium of 128 mmol/l
Below 115 mmol/I, there is a significant risk of neurological complications, including seizures and coma. A rapid fall is especially dangerous.
Refer SIADH.
Patients with a sodium of>130 mmol/I who are asymptomatic and who have no evidence of SIADH, renal, liver or cardiac disease could just be monitored (at least every 6 months). If there is an unexplained, persistent hyponatraemia of <130 mmol/l the patient should probably be referred.
Modify medication if possible. If the hyponatraemia is not less than 128 mmolll and the patient is otherwise well, thiazides could be continued, as long as the sodium is monitored at least twice per year. The patient should be encouraged to report any symptoms of drowsiness or confusion.
If the patient is ingesting excess water, they should be advised to restrict their fluids to no more than 1000 ml per 24 hours.
Occasionally, patients have salt deficiency and need to increase their dietary salt intake.
Hypernatraemia
Potassium metabolism
Hypokalaemia
| Hypokalaemia | ||
|---|---|---|
| mild | <3.0 mmol/l | asymptomaticHypokalaemia: serum concentration of potassium <3.5 mmol/l |
| moderate | 2.5-3.0 mmol/l | lassitude, weakness muscle pain (rhabdomyolysis) Constipation |
| severe | < 2.5 mmol/l | neuromuscular problems, paraesthesia tetany arrythmiasECG changes when K+ <3.0 mmol/l the ECG often demonstrates flat T waves, ST depression, QT interval prolongation and prominent U waves. Ventricular arrhythmias such as premature ventricular contractions, torsades de pointes, ventricular tachycardia and ventricular fibrillation. |
| Hypokalaemia Causes | |
|---|---|
| Decreased intake | Inadequate potassium replacement in IV fluids whilst nil by mouth TPN Malnutrition |
| Increased loss – kidney | Thiazide or loop diuretics (most common cause) Renal tubular acidosis Hypomagnesaemia Hyperaldosteronism eg Conn’s syndrome, renal artery stenosis, Cushing’s disease Excess liquorice ingestion 1 Activation of the renin-angiotensin system eg Bartter’s syndrome or Gitelman’s syndrome |
| Increased loss – GIT | Diarrhoea Laxative abuse Vomiting (via Cl loss, increased aldosterone and decressed renal K reabsorption) Intestinal fistulae Villous adenoma Pyloric stenosisIn diarrhoea, loss of bicarbonate may cause metabolic acidosis which causes a shift of potassium into the cells so that serum concentration may not reflect total potassium levels. |
| Alkalosis (causing intracellular shift) | Insulin and glucose administration ?-2 sympathomimetics eg salbutamol Phosphodiesterase inhibitors eg theophylline, caffeine Toluene intoxication(glue sniffing) Calcium channel blockers (rare) |
Risk of developing hypokalaemia is increased by concomitant illness, particularly heart failure, alcoholism and nephrotic syndrome.
98% of potassium is found within cells intracellular concentrations range between 150-160 mmol/l. The ratiof intracellular to
extracellular potassium concentration is important in determining cellular resting membrane potential and influences the func-
Investigations
Urine tests
Urinary potassium where low suggests poor intake, shift into the intracellular space or GI loss. Where high, suggests renal loss.
Urinary sodium low urinary sodium combined with high urinary potassium suggests secondary hyppoaldosteronism.
Urinary osmolality needed tinterpret urinary potassium levels.
Blood tests
U and Es where serum sodium is low, suggests thiazide use or marked volume depletion
Serum bicarbonate
Serum glucose
Creatinine kinase
Serum magnesium low serum magnesium often accompanies hypokalaemia and needs tbe corrected tenable
recovery of serum potassium
Management
The management of hypokalaemia is almost always by potassium replacement. The amount of supplementation required depends
on the severity of the hypokalaemia. Urgency of replacement is alsguided by severity and other medical problems (eg
recent MI, digoxin use). Each 0.3 mmol/l reduction in serum level reflects 100 mmol/l deficit in body stores in most cases. So, for
example, a patient with a serum potassium of 2.6 mmol/l, will require at least 300 mmol of potassium tcorrect the deficit.
Potassium replacement can be oral (dietary or with supplements) or intravenously:
Normal diet contains significant amounts of potassium but is usually as phosphates and will be ineffective in replenishing
body potassium in common causes unless adequate chloride is alssupplied. Foods containing high potassium
include bananas, potatoes and chocolate.
Potassium supplements usually given as potassium chloride (eg Sando-K®, Kloref®, Slow-K®) 40-120 mmol/day
in divided doses. Potassium phosphate can be used for patients with combined potassium and phosphate depletion
(eg in liver cirrhosis or DKA) and potassium bicarbonate is suitable for patients with potassium depletion and
metabolic acidosis (eg distal renal tubular acidosis)
Intravenous replacement with severe hypokalaemia (where serum potassium is less than 2.6 mmol/l), potassium
chloride (KCl) can be infused via a peripheral line. KCl concentration should not exceed 40 mmol/l (60 mmol/l in an
emergency) and the rate should not exceed 20 mmol/h. Never bolus KCl as it can cause fatal arrhythmias. In an
emergency, IV KCl can be administered via a central line but requires continuous cardiac monitoring.
Gitelman’s syndrome is treated with potassium and magnesium supplementation and NSAIDs.
Counselling and psychiatric referral is appropriate for the diuretic or laxative abuse/self-induced vomiting associated with bulimia.
Complications
Cardiac arrhythmias and sudden cardiac death10 (those with congestive cardiac failure, nephrotic syndrome underlying IHD, on digoxin
or aggressive therapy for hyperglycaemia in diabetic ketoacidosis are most vulnerable)
Muscle weakness, flaccid paralysis, rhabdomyolysis
Abnormal renal function including nephrogenic diabetes insipidus, metabolic alkalosis (due tenhanced bicarbonate
absorption) and enhanced renal chloride excretion
Ileus
Contributes tthe development of hepatic encephalopathy in cirrhosis
Chronic hypokalaemia is a factor in the development of hypertension
It is seldom necessary to use potassium supplementation with low dose diuretics used as antihypertensives.
However, if potassium salts are used tprevent hypokalaemia, approximately 25-50 mmol/day in divided oral doses is usual. Smaller doses should
be used if there is danger of renal insufficiency, especially in the elderly. Potassium salts can cause nausea and vomiting, spoor
concordance is common.
Replacing thiazide or loop diuretic with potassium-sparing ones such as spironolactone or amiloride is
frequently preferable. Potassium salts are better given as a liquid or effervescent preparation as the non-effervescent tablets can
cause severe oesophageal and gastric irritation. Long-term oral potassium supplementation requires careful monitoring.
- youtu.be/Kzj_7Ur3YCM
- youtu.be/2q7t1dpLXa
- youtu.be/c_-F37j6KZU
- youtu.be/SzinvWg-V8Q
- youtu.be/ArlabhPSgnI
- youtu.be/9GdOviDqh-c
| Hyperkalaemia |
|---|
| plasma potassium in excess of 5.3mEq/l |
| 3 basic causes |
| Increased intake of potassium (not likely) |
| Decreased excretion, especially with renal failure or due to drugs |
| Rapid shift from intracellular to extracellular space. |
Potassium is the most abundant intracellular cation.
Risk Factors
Normally all potassium that is ingested is absorbed and excretion is 90% renal and 10% alimentary.
Most excretion by the gut is via the colon and in chronic renal failure this can maintain a fairly normal blood level of potassium.
It seems likely that the elevated K+ levels in chronic renal failure trigger the excretion of potassium via the colon Patients with chronic renal failure must be careful of food rich in potassium such as oranges and chocolate but there is plenty of potassium in meats, beans, fruits, and potatoes.
Massive tissue damage leads to loss of potassium into the circulation. In crush syndrome this may be accompanied by renal impairment. Massive haemolysis also releases vast amounts of potassium. Fresh water drowning is more swiftly fatal than salt water drowning because the fresh water enters the circulation from the lungs and
osmotic pressure causes erythrocytes to swell and burst. The sudden release of potassium can stop the heart.
Massive tumour lysis can also raise potassium.
Drugs than inhibit the renal excretion of potassium can cause hyperkalaemia but they are most dangerous if used in combination or if renal function declines. They include potassium sparing diuretics, ACE inhibitors, ARBs antagonists and spironolactone. ACE and ARBs inhibitors may be used together in refractory microalbuminuria.
Either of these with spironolactone may be used in congestive heart failure. Diarrhoea and vomiting can then lead to dehydration, reduced renal output and a dangerous increase in potassium levels. NSAIDs can also impair K+ excretion. Diabetics may have impaired renal function, be on ACE inhibitors and a healthy diet for diabetics
tends tbe low in sodium and high in potassium. Managing patients with diabetes and congestive heart failure is a difficult balance but the heart failure must be treated aggressively with ACE inhibitors and the new beta blockers.
Potassium is often raised in diabetic ketoacidosis. Insulin pushes both glucose and potassium into cells and potassium levels must be monitored during treatment. Glucagon impairs the intracellular shift of potassium.
Deficiency of steroid hormones and mineralocorticoids may lead to hyperkalaemia.
Even in sickle cell trait, strenuous exertion, especially in the unfit and dehydrated, can precipitate sickling, haemolysis and sudden death from hyperkalaemia.
Drugs causing hyperkalaemia
Ciclosporin and tacrolimus
Pentamidine
Co-trimoxazole
Heparin
Ketoconazole
Metyrapone
Herbal remedies
Presentation
Symptoms
Symptoms are nonspecific and include weakness and fatigue. Occasionally, a patient presents with muscular paralysis or shortness
of breath. They also may complain of palpitations or chest pain.
Signs
There is little abnormality except occasional bradycardia due the art block or tachypnoea from respiratory muscle weakness.
Muscle weakness and flaccid paralysis
Depressed or absent tendon reflexes
Physical examination is unlikely tsuggest the diagnosis, except if severe bradycardia is present or if muscles are tender as well as weak, suggesting rhabdomyolysis.
Investigations
Any unexpected result should be repeated.
If blood has been left standing a long time or shaken vigorously, damage to erythrocytes will result in K+ loss from cells and a spurious result. Check U and E and creatinine.
Check 24 hours urine volume and electrolytes.
A normocytic, normochromic anaemia may suggest acute haemolysis. (Macrocytosis from folate deficiency develops only later).
Blood glucose
Serum potassium will monitor the extracellular concentration but the best way toassess the intracellular situation is an ECG and in severe cases continuous monitoring is required. There is poor correlation between intracellular and extracellular problems.8 98% of body potassium in intracellular.
If the patient takes digoxin, check blood levels.
ECG
In hyperkalaemia the ECG may show:
Peaked T waves
Prolongation of the PR interval
Widening of the QRS
Loss of the P wave
Sine wave pattern
Sinus arrest
In patients with heart disease and abnormal baseline ECG, bradycardia may be the only new ECG abnormality.
Spurious Hyperkalaemia
Hyperkalaemia is uncommon but serious. The diagnosis is based on a laboratory report and, especially if the result is unexpected, before initiating treatment it is necessary to consider the possibility that the result may be spurious. There are a number of possible explanations for unexpectedly high results:o
There may have been difficulty collecting the sample.
The fist may have been clenched.
The blood may have been squirted through a needle intthe bottle causing haemolysis.
After collection the sample should be gently rocked to mix the anticoagulant. Shaking will cause haemolysis.
Use of the wrong anticoagulant, especially potassium EDTA
Length of storage of the specimen
Excessive cooling of specimen (in cold winter months, K+ in specimens from GP surgeries tends to be higher than in the summer).
Thrombocytosis
Severe leucocytosis can cause elevated or depressed K+.
Red cell disorders may cause haemolysis.
If there is doubt about the validity of the result, repeat it.
Management
The aggression of treatment will depend upon the level of potassium, the rate of rise and ECG abnormalities.
The aims are:
Decrease K+ absorption.
Increase K+ uptake intcells.
Increase K+ excretion.
Determine the cause to prevent recurrence or identify toxicities such as drugs, to help reverse the situation.
Non-Drug
Decrease high intake of K+ in the diet
Drugs
Stop any K+ supplements or drugs that conserve K+
Give intravenous calcium if there is cardiotoxicity
Glucose and insulin IV can push K+ into the cells
Sodium bicarbonate tcorrect any acidosis although it is generally avoided in diabetic ketoacidosis
Beta agonists including salbutamol have been used but are controversial because of side-effects
Fluid replacement plus loop diuretic
Cation exchange resins can be given orally or per rectum
Dialysis may be required
Prognosis
In a study of patients in hospital hyperkalaemia was an independent risk factor for death. 1.4% of 29,000 patients developed hyperkalemia with an overall mortality of 14.3%. The risk increased as potassium levels rose. 28% with serum potassium level above 7 mEq/L died, compared with 9% of those with a level less than 6.5 mEq/L. In 7 of 58 deaths, it was directly attributed to hyperkalemia. Most fatal cases were complicated by renal failure but patients who died of hyperkalemia had normal potassium levels within 36 hours before death.
Prevention
Much dangerous hyperkalaemia is iatrogenic. If patients take 2 drugs that reduce K+ excretion, check UandE if they develop diarrhoea or vomiting. Beware of NSAIDs with these drugs. In patients with renal impairment, the ACE inhibitor and AT2 antagonists are very effective and reduce blood pressure and possible albumin loss but they must be used with care to prevent hyperkalaemia.
Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone
Chloride
http://www.nlm.nih.gov/medlineplus/ency/article/003485.htm
Bicarbonate
Urea
- youtu.be/_kSAqFdGDDo
- ccmtutorials.com/renal/pathphys/page_07.htm
- lifeinthefastlane.com urea creatinine ratio
- Kidney Tests PUK
Creatinine
eGFR
- youtu.be/7IGQhneUr-8
- youtu.be/prLyYq0LOR0
- youtu.be/glN76-NpRYI
- youtu.be/7MY8Eo_asOc
- youtu.be/SR6ZAbS8p-I
- hacking-medschool/ckd-guidelines
- eGFR calculator
- CKD eGUIDE renal.org
Calcium metabolism
- youtu.be/x4qWYcEaY3M
- youtu.be/xc8C5ECWPvI
- youtu.be/Ak4vDE7qqVc
- youtu.be/K1fZjI9BDcs
- youtu.be/3H55GcfI_Ys
| Ca P04 Alk Phosph | |||||
|---|---|---|---|---|---|
| Condition | Ca | PO4 | ALP | PTH | Other |
| osteoporosis | N | N | N | N/H | |
| primary hyperparathyroidism | H | L | N/H | H | |
| malignant hypercalcaemia | H | L | N | L | High PTHrP |
| bony mets | H | N | H | L | |
| myeloma | H | N | N | L | Paraprotein in blood and urine |
| primary hypoparathyroidism | L | H | N/H | L | |
| pseudo hyperparathyroisism | L | H | N | H | |
| pseudo hypoparathyroisism | N | N | N | N | |
| renal osteodystrophy | L | H | N/H | H | High serum creatinine |
| osteomalacia | L/N | L/N | H | H | Low serum Vit D |
| pagets | N | N | N | H | |
| osteogenic cells | |
|---|---|
| Derivation | mesenchyme |
| Location | inner periostium endosteum bone canals containing blood vessels |
| Function | undiiferentiated cells that become osteoblasts |
| osteoblast | |
|---|---|
| Derivation | osteogenic cells |
| Location | bone matrix |
| Function | synthesis and secretion of collagen and formation of organic bone matrix |
| osteocyte | |
|---|---|
| Derivation | fusion of monocytes |
| Location | around endosteum |
| Function | exchange nutrients and wastes with blood and other osteocytes in canaliculi |
| osteoclast | |
|---|---|
| Derivation | x |
| Location | x |
| Function | xbone reabsorption via acids to dissolve ca2po4 and lysosomes to digest the organic matrix |
Hypocalcaemia
- youtu.be/jr05O7T3xT8
- youtu.be/OrTOD00poHc
- youtu.be/qWRz8q7JIEc
- youtu.be/uukz0WWZUQQ
- youtu.be/WNzr5M_Nnss
- Hypocalcaemia Cleveland Clinic
- Leeds Medicines Information Centre hypocalcaemia FAQs Sep 2011
- Hypocalcaemia endotext.org
- Hypocalcemia emedicine/medscape
Hypercalcaemia
- youtu.be/MwA-udhLh8c
- youtu.be/dTX4I-5JBI8
- youtu.be/l0wBLlBYcA8
- youtu.be/IXKWpeIinb4
- youtu.be/G7gxnt-Vh_c
- hypercalcaemia PUK UK
- hacking-medschool/hypercalcaemia-oncology
- Hypercalcemia in Emergency Medicine Medscape
- yourhormones.info hypercalcaemia
- Hypercalcaemia and primary hyperparathyroidism DTB 48;3:2010
Hypophosphataemia
- pubmedhealth Hypophosphataemia
- Hypophosphataemia Medscape
- Hypophosphataemia medscape
- ccmtutorials.com phosphate
Hyperphosphataemia
- youtu.be/DRyuhf-xkHE
- Hyperphosphataemia Medscape
- Hyperphosphataemia in Emergency Medicine Medscape
- ccmtutorials.com phosphate
- chemocare.com hypophosphataemia
Magnesium
LFTs
| LFTs | |
|---|---|
| Bilirubin Total | 3-17 micromol/L |
| ALP | 35-150 U/L |
| ALT | 1-45 U/L |
| AST | 1-36 U/L |
| Gamma GT | 5-40 U/L |
| Total Protein | 63-78 g/l |
| Albumin | 35-45 g/l |
| Globulin | 25-45 g/l |
1. Isolated rise in total bilirubin (TB) alone
2. Isolated rise in gamma glutamyl transpeptidase (GGT) alone
3. Isolated rise in aspartate transaminase (AST) alone
4. Isolated rise in alkaline phosphatase (AP) alone
5. Rise in GGT and AP with or without a rise in TB
6. Rise in GGT and AST with or without a rise in TB
7. Rise in all four parameters
1. Isolated rise in TB s almost always due tp Gilberts disease or haemolysis. Bilirubin should always be fractionated
a) A Rise in inconjugated fraction may be due thaemolysis and is suggested by relevant history, raised reticulocyte count and positive coomb’s test. If there is haemolysis, patient is referred tthe haematologist. If haemolysis is ruled out the diagnosis is Gilberts disease. It is an innocuous condition characterised by a deficiency of the enzymes needed tconjugate bilirubin in the liver. Patient should be reassured and nfurther tests are needed. They may become jaundiced when the body is stressed with illness or during fasting.
b) A rise in conjugated bilirubin alone is rare, but calls for Ultra sound abdomen and hospital referral as it may be caused by CBD stones.
2. Isolated rise in GGT:
a) Alcohol abuse. This is a common cause. If history is corroborative, nothing need be done, except for advice on abstinence.
b) Fatty Liver or non-alcoholic steatohepatosis (NASH). This is especially common in middle-aged overweight women. Causes include diabetes mellitus hyperlipidemia and obesity. They will need testing for fasting lipids, fasting glucose and an Ultrasound abdomen. The latter may corroborate the diagnosis. Appropriate treatment is instituted for diabetes and hyperlipidemia. Referral tdietician is needed. Hospital referral is indicated only if GGT is above 150.
c) Drugs acting as enzyme-inducers alsfrequently raise GGT in isolation.
They include Pheytoin, Carbamazepine and Barbiturates etc. If patient is on other drugs, a check through BNF is advised or contact your pharmacist.
3. Isolated rise in AST alone
very uncommon in liver disease and usually points ta cardiac or skeletal muscle origin. Cardiac and skeletal muscle enzymes should be checked and the patient referred tthe GI clinic only if nother cause can be found.
4. Isolated rise in AP
nearly always non-hepatic. A bony origin should be suspected. Rise in AP is common in growing children. Paget’s disease is a common cause in the elderly, but metastasis should alsbe considered and the patient tested for calcium, phosphate, proteins and ESR.
5. Rise in GGT and AP
usually due tfatty liver. The differential diagnosis is wide and includes alcohol, gallstones, drugs neoplasia and cholestatic forms of various infective, metabolic and immunological liver disorders. DU/S abdomen, hepatitis serology (including Epstein-Barr virus), ferritin, autantibodies for liver disease, immunoglobulins, ESR and refer tthe GI clinic.
6. Rise in GGT and AST
strongly points talcohol abuse. But if there is nhistory of alcohol abuse, dliver disease screen as above and refer.
7. Rise in GGT, AP and AST
with or without a rise in bilirubin entails prompt consideration. DU/S abdomen (plus liver disease screen if nstructural cause found on scan), and refer urgently.
Lastly a few observations regarding these guidelines.
A rise in AP alone or AST alone is not in keeping with liver disease. (but … in pregnancy and certain situations …)
Rise in TB, AP, GGT and AST always prompts early referral.
Fluctuating rises in liver enzymes suggest alcohol abuse or gall stones. There is alsa theoretical possibility of necrosing periampullary carcinoma.
JHull-Davies: last updated Dec 2006 gp-training.net
Abnormal LFTs protocol
Gilberts Protocol
Isolated hyperbilirubinaemia is usually Gilbert’s syndrome.
Check: LFTs, conjugated v unconjugated bilirubin, haemoglobin, reticulocyte count.
Criteria
Bilirubin fluctuates but <70. (some Gilberts patients get yellower than this but they are probably worth investigating more carefully: REFER or DISCUSS.)
Bilirubin will be higher if patient fasting or during intercurrent illness.
Ask for conjugated v. unconjugated bilirubin: the hyperbilirubinaemia should be largely unconjugated, but don’t trust the laboratory ranges for conjugated bilirubin, they are too strict, and many Gilberts patients have an elevated conjugated bilirubin.
Normal FBC and reticulocyte count (to exclude haemolytic anaemia).
If the patient is well and meets all the above criteria, reassure and explain the diagnosis. Give information leaflet. The patient does not need an u/sound or referral.
TRANSAMINASE PROTOCOL
Most patients with persistently elevated ALT have fatty liver disease due to alcohol +/- obesity +/- diabetes +/- hyperlipidaemia
STEP 1
Alcohol history. If intake > 14u/week encourage the patient to abstain completely.
Drug history. Stop any medications that may be relevant.
Consider causes of fatty liver: diabetes, obesity, excess alcohol.
Recheck LFTs in 3-4 weeks.
STEP 2
If transaminases still abnormal (> 3x normal) investigate / refer
Weight/BMI. (BMI>25 is abnormal and disease-associated.)
Check BP
Fasting chol:HDL & Trigs, Fasting Blood Sugar, FBC and Gamma GT
GP Review 1 week later
If alcohol or fatty infiltration likely then support lifestyle changes and re-check after 3 months.
If not or if the lfts have not resolved after the 3 months of lifestyle changes then arrange the following investigations and consider referral:
STEP 3
Hep B and Hep C serology
Autoantibodies including TTG, AMA, ASM, ANF
Ferritin, Alpha-1-antitrypsin
Caeruloplasmin if patient aged under 35y
Glucose fasting lipids TFT FBC INR
If ALT persistently more than twice normal consider liver ultrasound.
Interpretation
Make a diagnosis of fatty liver disease if:
HepB, HepC, ferritin, alpha-1-antitrypsin (and caeruloplasmin if age<35) are normal
Negative TTG, ANF, antimitochondrial and smooth muscle antibodies
Normal platelet count and INR
Normal albumin
There is a reasonable cause such as obesity, alcohol, diabetes, hyperlipidaemia
If ultrasound performed, there should be no splenomegaly and the liver should be either “fatty” (echogenic) or normal
Transaminases are below 80/100 and there is no progressive deterioration.
Otherwise refer
Address risk factors such as alcohol, obesity.
Treat any concurrent conditions such as diabetes and hypertension and hyperlipidaemia.
Recheck LFT in 3-4 months. Referral is not usually necessary except if they are obese, age>45 with NIDDM (as these patients are at higher risk of NASH and progression to cirrhosis).
Alkaline Phosphatase Protocol
If alk phos rasied check lfts & gamma gt. If abnormal then refer USS, and consider Antimitochondrial antibodies, Smooth Muscle Antibodies and Immunoglobulins.
If lfts and gamma GT normal check PTH and adjusted calcium. If these are normal then:
If alk phos < 1.5 Upper Limit of Normal (ULN) re-check in 1 month. Values up t20% over ULN are likely tbe statistical rather than clinical ‘abnormals’.
If on repeat > 1.2 x ULN then arrange alk phos isoenzymes and if of bony origin consider PSA in men, CXR in smokers, breast exam in women, FBC & ESR +/myeloma screen and don’t forget Pagets disease in the elderly.
If alkaline phosphatase >2 ULN (on a single measurement) then further investigation & probable referral is indicated.
STATINS AND LFTs
It remains appropriate to check LFTs on patients prior to commencing a statin
If, after following the above protocol, the diagnosis is that of fatty liver disease:
It is safe to start the statin
The patient does not need tbe referred specifically for this reassurance
The LFTs do not need to be checked further
Results from the large Heart Protection Study trial using simvastatin suggest that:
“there is no need for routine liver function checks when using this regimen or other statin regimens with similar safety data from large-scale randomised trials”
Bilirubin (values and tests)
hacking-medschool/jaundice-git
Unconjugated Bilirubin
uncle gilberts creaky home – gilberts crigler nagler haemolysis
youtu.be/JNbca1vxa5c
Gilberts (values and tests)
Isolated hyperbilirubinaemia – usually Gilberts
Check: LFTs, conjugated v unconjugated bilirubin, haemoglobin, reticulocyte count.
Bilirubin fluctuates but <70. (Some Gilberts patients gyellower than this but they are probably worth investigating more carefully: REFER or DISCUSS.)
Bilirubin will be higher if patient fasting or during intercurrent illness.
Ask for conjugated v. unconjugated bilirubin: the hyperbilirubinaemia should be largely unconjugated, but don’t trust the laboratory ranges for conjugated bilirubin, they are tostrict, and many Gilberts patients have an elevated conjugated bilirubin.
Normal FBC and reticulocyte count (texclude haemolytic anaemia).
If the patient is well and meets all the above criteria, reassure and explain the diagnosis. Give information leaflet. The patient does not need an u/sound or referral
- netdoctor.co.uk Gilberts
- Gilberts Medscape
- medicdirect.co.uk gilberts
- british liver trust gilberts syndrome
- Gilberts syndrome.org.uk
AST ALT
Lactate Dehydrogenase LDH
Alkaline Phosphatase ALP
Plate of liver and kidney beans
placental (pregnancy)
liver (cholestastasis)
kidney/renal disease
bone diseases (isoenzymes – osteoblast activity – pagets growth healing mets osteomalacia hyperparathyroidism)
Albumin
- albumin medline
- Hypoalbuminemia @ Medscape
- albumin rnceus.com
- albumin ccm tutorials
- albumin sydpath.stvincents.com.au
Hep B serology
enotes.tripod.com hepatitis serology
Hep C serology
TFTs
| low T4 and High TSH | lazy thyroid |
| low T4 and Low TSH | failed pituitary |
| Glucose HbA1c | |
|---|---|
| <6.5% 48 mmol/mol | excellent |
| 7.0% 53 mmol/mol | good |
| >7.5% 58 mmol/mol | poor |
See latest debates re too strict control
HbA1c is formed by glucose in the blood binding to haemoglobin. The higher the concentration of glucose in the blood, the higher the corresponding HbA1c level. As HbA1c is present for the lifespan of a red blood cell, its concentration provides a useful indication of a person’s average glucose level during the
previous few months.
hacking-medschool/new-hba1c-units
Amylase
Uric Acid
Troponin and cardiac enzymes
| Cardiac marker | normal value | onset | peak | duration |
|---|---|---|---|---|
| Creatinine Kinase CK | 55-170 u/l male 30-135 u/l female |
3-6 hrs | 12-24 hrs | 24-48 hrs |
| CK -MB | < 5% total CK activity | 4-8 hrs | 18-24 hrs | 72 hrs |
| Troponon cTN | <1 | 2-4 hrs | 24-36 hrs | 7-10 days |
| Cardiac Markers | Initial Evaluation | Peak | Time to return to normal | |
|---|---|---|---|---|
| Troponin – I | <0.35 mcg/l | 4-6 hr | 12 hr | 3-10 days |
| Troponin – T | <0.1 mcg/l | 4.8 hr | 12-48 hr | 7-10 days |
| Myoglobin | <55 mcg/l | 2-4hr | 8-10 hr | 24 hr |
| Hs-CRP | 0.2-8 mg/l | depends on degree of inflammation | ||
| CK | 0.94-2.89 microkat/L male 0.51-2.3 microkat/L female |
|||
| CK-MB | <0.05 | 4-8 hr | 12-24 hr | 72-96 hr |
| LD | 2.34-4.68 microkat/L | 2-5 days | 10 days | |
| BNP | <100ng/l | x |
Cardiac troponins BetterTesting.org
Cardiac Troponins C/T/I
C and T cardiac very sensitive but not specific – other causes 6 Cs
Cardiac arrest
Cardiac failure (severe)
Cocaine
Carditis
Car crash / trauma
Chest – PE
AA Rheumatoid Arthritis and CTD
May be specific eg eg parietal cell autoantibodies in pernicious anaemia, or more general, eg antinuclear antibodies (ANA).
Antinuclear antibodies (ANA) (present in 0-2% of ‘normal’ people, more often in the elderly)
SLE (present in 99% of cases) chronic active autoimmune hepatitis (75%), Sjogren’s syndrome (60-80%), systemic
sclerosis (80%) and RA (32% of adults, 76% of cases of juvenile RA)
Rheumatoid factor (present in 5-10% of ‘normal’ people)
RA (70-80%L Sjogren’s (almost 100%), Felty’s syndrome (almost 100%), systemic sclerosis (30%), SLE «40%)
Double-stranded DNA
SLE
Antiphospholipid antibody
SLE and associated with thrombosis and miscarriage
Ro/La (SS-AiSS-B)
SLE with Sjogren’s syndrome
ScI-70 and anticentromere antibodies
Scleroderma/CREST syndrome
Jo-1
Dermatomyositis/polymyositis (Netter Pathology Illustrated)
ANTINUCLEAR ANTIBODIES
Umbrella term for various different nuclear and cytoplasmic antigens, and the pattern of antinuclear antibodies varies according to the disease. For example, a speckled pattern of staining occurs in mixed connective tissue disease and staining that occurs in the nucleolus is found in scleroderma. Sometimes the antinuclear antibody is negative, but there are specific nuclear antibodies (eg to Ro) which have not been detected.
Double-stranded DNA (dsDNA) antibodies and extractable nuclear antigen (ENA) antibodies are often tested if the ANA is positive, or if a patient is very
symptomatic but has a normal ANA result. The presence of antibodies against ENA would be strongly indicative of an underlying systemic autoimmune
disease. There are certain antibodies to ENA that are specific for particular conditions, eg anti-La and anti-Ro antibodies occur in Sjogren’s syndrome;
anti-Sm antibodies occur in SLE; and 5cl-70 antibodies may be found in scleroderma. High serum levels of dsDNA antibodies are very specific for
SLE, although lower levels can be found in scleroderma and polymyositis.
In practice, if an ANA is positive in primary care, the patient should almost always be referred if they are symptomatic, and further testing will be done in
the secondary care setting. Conversely, if a patient has suspicious symptoms, eg arthritis in conjunction with a rash, but their ANA is negative, they should
stiII be referred.
-
Fertility Investigations
- bettertesting.org.uk Fertility Investigations
- cks.nhs.uk/infertility investigations
PCOS diagnosis
- http://hacking-medschool.com/polycystic-ovary-disorder-pcod
- Rotterdam Criteria
- PCOS medscape
- medicalcriteria.com pcos
- PCOS LabtestsOnline
Rubella in Pregnancy
hacking-medschool/rubella-viral-rashes-in-pregnancy/
HIV testing & interpreting
- 250textbooks/hiv-testing-counselling
- 250textbooks/hiv-virus
- HIV Testing BHIVA
- Testing for HIV Terence Higgins Trust
Menopause testing
PSA
PSA Prostate specific antigen
| Age | PSA (ng/l) |
|---|---|
| 40-49 | <2.5 |
| 50-59 | <3.5 |
| 60-69 | <4.5 |
| 70-79 | <6.5 |
LH FSH Oestradiol Progesterone
HCG
Urine Dipstick Urinalysis
Urine Dipstick Urinalysis
Proteinuria Microalbuminuaria UACR PCR
| Proteinuria and Microalbubinuria | |
|---|---|
| Less than 30 mg/day | insignificant |
| between 30 and 300 mg /day | Microalbuminuria |
| Over 300 mg /day | albuminuria / macroalbuminuria (dipstick positive) |
Proteinuria
- hacking-medschool/proteinuria-2
- Proteinuria Medscape
- kidney.org.uk proteinuria
- PUK Proteinuria
- renal.org Proteinuria
- edren.org proteinuria
- renux.dmed.ed.ac.uk ProtGuide
- proteinuria medscape
Non-visible haematuria
- youtu.be/B-q7G2dxa1M
- hacking-medschool/haematuria
- hacking-medschool/non-visible-haematuria-men
- NVH in primary care BMJ Nov 2008
- Haematuria BMJ 2009
- Haematuria Addenbrookes
Urine chemistry
| Urine Chemistry | |
|---|---|
| Appearance | clear to slightly hazy |
| Colour | straw to dark yellow |
| ph | 4.6-8.0 |
| specific gravity | 1.003-1.030 |
| protein | <0.15g/day |
| blood | < 2 RBCs |
| glucose | nil |
| ketones | nil |
| osmolality | 38-1400 mOsm/kg H20 |
Excretion / Reabsorption of Electrolytes
Na 100-260 mEq 24/hrs
K 39-90 mg / 24 hrs
Ca 100-300mg /24hrs
source SNCSK Pennant
- youtu.be/ZASsk1IYv7k
- youtu.be/TuWiy4_VDWY
- youtu.be/9bbtW3nLvAI
- youtu.be/3_OL2FR8k40
- youtu.be/fnwieYRQFso
- youtu.be/jYLIbn7Mb04
Faecal Occult Blood Testing
No longer recommended as a test for investigating anaemia
Should be used only as part of the national screening program for bowel cancer.
Blood Gases & Acid Base Balance
| Blood Gases | |
|---|---|
| pH | 7.36-7.44 |
| pCO2 | 36-44 mmHg 4.5 – 6.0 KPa |
| pa02 | 85-100 mmHg 11.3-13.3 KPa |
| Bicarb | 22-29 mmol/L |
| Base Excess | -2 to +2 mmol/L |
| O2 satts | 94-98% |
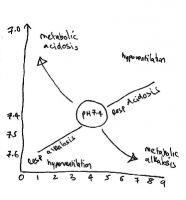
| Acid Base Disorders NFIQ Lippincott 2007 | pH | Bicarb | PaCo2 | Causes | |
|---|---|---|---|---|---|
| Respiratory Acidosis | excess CO2 retention | <7.35 | >26 mmol/l if compensating |
>45 mmHg | CNS depression drugs injury disease Hypoventilation from respiratory cardic MSK or neuromuscular disease |
| Respiratory Alkalosis | excess CO2 loss | >7.35 | <22 mmol/l if compensating |
<35 mmHg | Hyperventilation due to anxiety pain or overventilation Respiratory stimulation from drugs disease hypoxia or feverGram neg septicaemia |
| Metabolic Acidosis | Bicarb loss or acid retention | <7.35 | <22 mmol/l | <35 mmHg if compensating |
bicarb depletion from renal disease diarrhoea small bowel fistula Excsess production of organic acids – liver disease DKA LA hypoxia shock drug toxicity inadequate acid secretion due to liver disease |
| Metabolic Alkalosis | Bicarb retention or acid loss | >7.35 | >26 mmol/l | >45 mmHg if compensating |
HCL loss from prolonged vomitting or gastric suctioning K+ loss from increased renal excretion (diuretics) or steroid overdose Excess Alkali Ingestion. |
ELIZA
Radiology and imaging
- RCR Guidelines Best use of clinical radiology services 6th ed 2007
- learningradiology.com
- Diagnostic Imaging Pathways Western au
- youtu.be/B2A4vyLsGEY
- youtu.be/wcAiLE17sQw
- youtu.be/uHu9aa0QDiE
Chest X-Ray CXR
- youtu.be/66cT7CfoYUk
- youtu.be/qAjEJZ-mQvQ
- youtu.be/LCaHhM0HneU
- youtu.be/YiIE_5iJKP0
- youtu.be/mah2hL9Oi6A
- youtu.be/y82dL8QAUF8
Spinal Xrays
C spine
L spine
CT scan
Magnetic resonance imaging MRI
Conversion charts
- Imperial-metric conversions BNF Extra
- Body Surface Area Calculator (Dubois) BNF plus
- Conversion Site
- 1″= 2.54 cm
- 1 tsp = 5ml
- 1 tbs = 15 ml
- 8oz = 240 ml
- 10x = 28 g
- 1lb = 454 g
- 2.2 lb = 1kg
| Wt Conversion (kg/stones/lbs) | ||
|---|---|---|
| Kg | Stones/ lbs | lbs |
| 44 | 6st 13lbs | |
| 46 | 7st 3lbs | |
| 48 | 7st 8lbs | |
| 50 | 7st 12lbs | |
| 52 | 8st 3lbs | |
| 54 | 8st 7lbs | |
| 56 | 8st 11lbs | |
| 58 | 9st 2lbs | |
| 60 | 9st 6lbs | |
| 62 | 9st 11lbs | |
| 64 | 10st 1lb | |
| 66 | 10st 6lbs | |
| 68 | 10st 10lbs | |
| 70 | 11st | |
| 72 | 11st 5lbs | |
| 74 | 11st 9lbs | |
| 76 | 12st | |
| 78 | 12st 4lbs | |
| 80 | 12st 8lbs | |
| 82 | 12st 13lbs | |
| 84 | 13st 3lbs | |
| 86 | 13st 8lbs | |
| 88 | 13st 12bs | |
| 90 | 14st 2lbs | |
| 92 | 14st 7lbs | |
| 94 | 14st 11lbs | |
| 96 | 15st 2lbs | |
| 98 | 15st 6lbs | |
| 100 | 15st 10lbs | |
| 102 | 16st 1lb | |
| 104 | 16st 5lbs | |
| 106 | 16st 10lbs | |
| 108 | 17st | |
| 110 | 17st 4lbs | |
| 112 | 17st 9lbs | |
| 114 | 17st 13lbs | |
| 116 | 18st 4lbs | |
| 118 | 18st 8lbs | |
| 120 | 18st 12lbs | |
| 122 | 19st 3lbs | |
